|
CopA3를 이용하여 피부 염증에 대하여 연구를 하였다. 산화질소와 cytokine의 생산은 면역세포의 대표적인염증인자이다. 세포는 LPS 처리 후 한 시간 뒤에 CopA3를 처리하였다. 세포 독성이 나타나지 않는 농도인 5, 25, 50, 100 μg/ml를 사용하였다. CopA3는 NO, TNF-α, IL-1β, IL-6, iNOS, COX-2의 생성을 저해 시켰다. iNOS와 COX-2 역시 100 μg/ml의 농도에서 각각 54%, 65%가 저해가 되었다. 게다가 CopA3는 염증성 사이토 카인인 TNF-α, IL-1β, IL-6의 생성을 감소 시켰다. 이러한 결과로 CopA3는 염증 예방과 치료에 효과적임을 확인 할 수 있었다.
|
서 론
염증 반응은 생체나 조직에 물리적 작용이나 화학적 물질, 세균 감염 등의 어떠한 기질적 변화를 가져오는 침습이 가해질 때 그 손상 부위를 수복 재생하려는 기전이며[8], 염증 반응을 유발시키는 매개 물질로 활성산소(free radicals), 산화질소(nitric oxide, NO), prostaglandin 등이 있다[24]. 일단 자극이
가해지면 국소적으로 염증성 성분과 같은 혈관 활성 물질이 유리되어 혈관 투과성이 증대되면서 염증을 유발하지만 지속적인 염증반응은 도리어 점막손상을 촉진하고, 그 결과 일부에서는 여러 질환을 발생시키는 원인이 된다[22]. 대식세포는 선천면역뿐만 아니라 획득면역 등 다양한 숙주반응에 관여하여 항상성 유지에 관여하는 것으로 알려져 있으며, 염증반응시에는 nitric oxide (NO)와 cytokine을 생산하여 감염초기에 생체방어에 중요한 역할을 한다[7]. NO의 합성 효소는 eNOS,
nNOS, iNOS가 있는데, 이 중 iNOS는 칼슘의 농도에 상관없이 대식세포에서 TNF-α, IL-1β, IFN-γ와 같은 염증성 자극에 의해 유도되는 것으로 알려졌으며, 특히 LPS 처리시 다량 생성되는 것으로 알려졌다. TNF-α는 염증과 면역반응의 중요한 매개물질이며, 다양한 세포의 성장과 분화를 조절하는 것으로 알려졌다. 또한 세포에 독성을 일으키고, 혈관 형성, 골 흡수, 혈전 생성을 촉진하고 lipogenetic 대사를 억제하는 것으로 알려졌다[1].
곤충은 동물군 중에서 가장 많은 생물군으로서, 전 세계적으로 180만 종이 서식하고 있고, 우리나라에서만 1만 2천 종이나 서식하는 것으로 알려져 있다. 곤충은 생물 다양성이 매우 풍부하며, 환경에 따라서 이 곤충의 다양성이 결정되고 이에 관련된 여러 가지 곤충 유래의 생리활성 물질의 양과 질이 다양하게 변화된다. 최근에는 새로운 기능성을 갖는 약용곤충 을 발견, 상용화, 사육화, 보급화 함으로서 농가의 수익을 보장하고 새로운 식의약품으로 개발이 활발히 진행되고 있는 추세
이다[17, 18].
소똥구리류는 목초지에서 우분을 지하로 운반하는 작용을 하며, 이러한 우분을 발생원으로 하여 파리의 발생 억제, 토양의 비옥화와 물리성 개선, 우분과 함께 배설된 가축내부기생충 방제 등의 효과가 있다[2, 3]. 또한 소똥구리가 활동한 토양분산분석(analysis of variance, ANOVA) 후 tukey test로 다중비교를 실시하였다.
결과 및 고찰
Raw 264.7 cell에 대한 독성
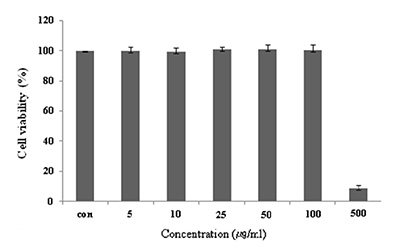
마우스 대식세포인 Raw 264.7 cell에 대한 CopA3의 세포독성을 확인하기 위하여 MTT assay를 수행하였다. CopA3를 5, 10, 25, 50, 100, 500 μg/ml 농도로 24시간 동안 처리한 결과 100 μg/ml의 농도까지는 독성이 나타나지 않았지만, 500 μg/ml의 농도에서는 세포의 생존율을 95% 감소 시켰다(Fig. 1). CopA3는 100 μg/ml 이하의 농도에서는 세포독성이 낮아 세포의 생존율에 영향을 주지 않는다는 사실을 확인할 수 있었다. 즉, CopA3의 항염증 효과가 단순한 세포의 사멸에 의한 세포 염증성 매개물질의 생성억제가 아니라 CopA3의 고유한 효과라는 점을 의미한다.
Fig. 1. Cell viability of CopA3 on Raw 264.7 cell. Raw 264.7
cells were treated with 5,10, 25, 50, 100, 500 μg/ml of
CopA3 dissolved in media for 1 hr prior to the addition
of LPS (1 μg/ml), and the cells were further incubated
for 24 hr. Data represent the mean±S.D. with eight separate
experiments. Data represent the mean±S.D. with
three separate experiments.
Nitric oxide (NO) 생성억제 효과
NO는 체내 방어기능, 신호전달 기능, 신경독성, 혈관 확장 등의 다양한 생리기능을 가지고 있으며, 3종류의 NOS (neuronal NO synthase (nNOS), endothelial NO synthase (eNOS), inducible NO synthase (iNOS))에 의해 합성된다. 이들 NOS 중 iNOS에 의한 NO 생성이 절대적으로 많으며 이는 병리적으로 중요한 작용을 한다[23]. LPS 자극에 의해 발현된 iNOS는 많은 양의 NO를 생성하게 되며, 이에 의한 세포독성은 염증반응, 세포의 돌연변이 및 종양발생 등에도 관여하는 것으로 알려져 있다. 염증반응과 관련된 조직 손상에서 NO와 iNOS의 발현이 증가되어 있음이 보고되어 있다[15, 16, 21].
NO생성에 대한 CopA3의 효과를 알아보았다. 생성된 NO양을 griess 시약을 이용하여 세포배양액 중에 존재하는 NO2의 형태로 측정하였다. 그 결과 Raw 264.7 cell에 LPS를 처리한 후 CopA3를 처리한 NO 생성량의 변화는 Fig. 2에 나타내었다. LPS 처리 후 NO 생성량은 정상세포에 비하여 약 4배 이상 증가되었다. CopA3는 50 μg/ml의 35% 이상의 감소율을 나타내었으며, 100 μg/ml의 농도에서는 80% 이상의 NO 생성 저해를 일으키는 것을 확인할 수 있었다.
Fig. 2. Inhibitory effects of CopA3 on the production of nitric
oxide Raw 264.7 cells. Raw 264.7 cells were cultured
with LPS (1 μg/ml) in the presence or absence of CopA3
for 24 hr to determine the level of NO. Nor: LPS not
induced group, Con: LPS induced group. The data represent
the mean±SD of three separate experiments
(significant as compared to control. *p<0.05).
항균활성실험에 쓰인 미생물은 Staphylococcus aureus(황색포도상구균), Staphylococcus epidermidis(피부상재균), Escherichia coli(대장균), Propionibacterium acnes(여드름원인균), Streptococcus mutans(충치원인균), Pityrosporum ovale(비듬유발균), Candida albicans (칸디다증균)등 총 7가지이다. 평가방법은 디스크확산법(Disk paper diffusion)을 시행하여 Clear zone을 확인하는 방법으로 항균활성을 평가하였다.
TNF-α, IL-1β, IL-6 생성 억제 효과
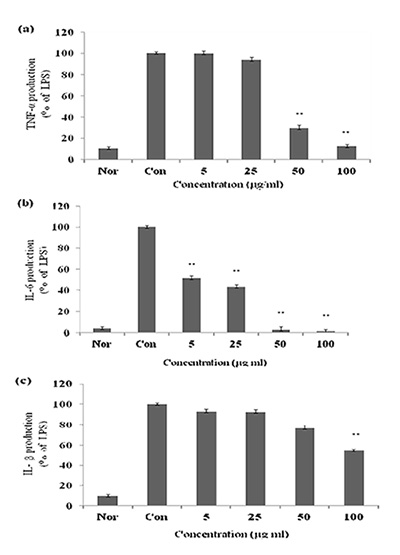
대식세포는 동물 체내 모든 조직에서 분포하는 면역세포로서 세균이나 이물질을 탐식 제거하며, IL-1β, IL-6, TNF-α 등의 염증 매개 물질들을 분비하여 초기 염증 반응에 주요 역할을 담당 한다[7, 13]. 특히, TNF-α는 염증반응에 있어서 중요한 역할을 하며 macrophage와 mast cell 등에서 분비되며, LPS반응의 주요 매개체로서 내재면역에 있어서도 중요한 역할을 하며 만성 염증 반응과도 관련되어 있다[14]. IL-1β는 T-cell의 활성화, B-cell의 성숙, NK cell의 activity를 활성화 하며, IL-6 는 림프구를 활성화시켜 항체생산을 증가시키는 것으로, IL-6의 level은 염증성 병변에서 항상 증가하는 것으로 보고되고 있다[4]. 본 실험에서 LPS는 TNF-α, IL-1β, IL-6의 생성을 증가시켰지만 CopA3 처리한 결과 TNF-α는 60% 생성 억제를 IL-1 β, IL-6는 각각 50 μg/ml의 농도에서 50%, 55%의 생성 억제 효과를 나타내었다(Fig. 3). 일반적으로 LPS는 macrophage에 작용하여 TNF-α, IL-1β, IL-6의 생성 분비를 촉진시켜 염증반 응을 유도하지만 CopA3는 이 세 가지의 cytokine을 유의성 있게 억제하였다.
Fig. 3. Effect of CopA3 on the production of cytokines stimulated by
LPS. Production of TNF-α (a) IL-6 (b), IL-1β (c) were measured
in the medium of Raw 264.7 cells cultured with LPS (1 μg/ml)
in the presence or absence of CopA3 for 24 hr. The amount
of TNF-α was measured by immunoassay as described in materials
and methods. Nor: LPS not induced group, Con: LPS
induced group. Data represent the mean±S.D. with three separate
experiments. One-way ANOVA was used for comparisons
of multiple group means followed by t-test (significant
as compared to control. *p<0.05, **p<0.01).
iNOS, COX-2의 단백질 발현 저해 효과
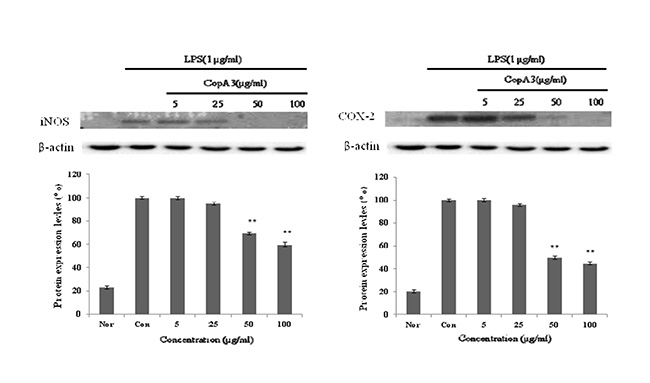
iNOS는 평소에는 세포 내에 존재하지 않으나 일단 유도되면 장시간 동안 다량의 NO를 생성하며, 생성된 NO는 혈관투과성, 부종 등의 염증 반응을 촉진시킬 뿐만 아니라 염증매개체의 생합성을 촉진하여 염증을 심화시키는 것으로 알려져 있다[12, 20]. NO의 생성에는 NO synthase (NOS)가 작용하게 되는데, constitutive NO synthase (cNOS)와 iNOS 중 자극에 유도된 iNOS의 경우 오랜 기간 동안 다량의 NO를 생성하고, 생성된 NO는 guanyl cyclase의 활성과 동시에 세포독성을 나타내게 된다. 따라서 NO로 유도 되어진 RAW 264.7 cell에서 iNOS의 protein level의 감소를 확인으로서 항염증 효과를 기대할 수 있으며, 또한 세포와 같은 macrophage 따른 monocyte에서 TNF-α, IL-6 와 같은 proinflammatory cytokine을 증가시키는 요인 중 하나인 COX-2의 protein level의 감소를 이끌어 냄으로서 항염증 효과를 기대할 수 있다[19]. CopA3에 의한 NO 생성 저해 기전을 확인하기 위해 western blot을 실시하여 iNOS의 단백질 발현을 측정 하였으며, western blot을 이용하여 COX-2의 발현을 측정하였다. 그 결과 LPS에 의해 증가된 iNOS와 COX-2의 단백질 발현량이 농도 의존적으로 유의성 있게 감소되었다. β-actin의 band density 비율에 따라 iNOS 단백질 생성을 41%와 COX-2의 단백질 발현을 56% 저해함을 확인 하였다(Fig. 4). 이를 통해 CopA3는 iNOS, COX-2 의 생성을 저해하는 것을 확인할 수 있었다.
Fig. 4. Inhibitory effects of CopA3 on the protein levels of iNOS and COX-2 in Raw 264.7 cells. Raw 264.7 cells (5x105 cells/ml)
were pre-incubated for 24 hr, and the cells were stimulated with lipopolysaccharide (1 μg/ml) in the presence of complex
extracts sample (5, 25, 50, 100 μg/ml) for 24 hr. Nor: LPS not induced group, Con: LPS induced group. Data represent
the mean±S.D. with three separate experiments. One-way ANOVA was used for comparisons of multiple group means followed
by t-test (significant as compared to control. *p<0.05, **p<0.01).
감사의 글
본 논문은 농촌진흥청 동물유전체육종사업의 일환으로 수행된 연구결과의 일부이며 이에 깊은 감사를 드립니다.
References
1. Aggarwal, B. B. 2003. Signaling pathways of the TNF superfamily:
a double-edged sword. Nat Rev Immunol 3, 745-756.
2. Bang, H. S., Lee, J. H., Kwon, O. S., Na, Y. E., Jang, Y. S.
and Kim, W. H. 2005. Effects of paracoprid dung beetles
(Coleoptera:Scarabaeidae) on the growth of pasture garbage
and on the underlying soil. Applied Soil Ecology 29, 165-171.
3. Bornemissza, G. F. and Williams, C. H. 1970. An effect of
dung beetle activity on plant yield. Pedobiologia 10, 1-7.
4. Delgado, A. V., McManus, A. T. and Chambers, J. P. 2003.
Production of tumor necrosis factor-alpha, interleukin 1-beta,
interleukin 2, and interleukin 6 by rat leukocyte subpopulations
after exposure to substance. Neuropeptide 37,
355-361.
5. Fincher, G. T. 1981. The potential value of dung beetles in
pasture ecosystems. J Ga Entomol Soc 16, 316-333.
6. Hwang, J. S., Lee, J., Kim, Y. J., Bang, H. S., Yun, E. Y.,
Kim, S. R., Suh, H. J., Kang, B. R., Nam, S. H., Jeon, J. P.,
Kim, I. and Lee, D. G. 2009. Isolation and characterization
of a defensin like peptide (Coprisin) from the dung beetle,
Copris tripartitus. Int J Pept DOI: 10.1155/2009/136284.
7. Higuchi, M., Higashi, N., Taki, H. and Osawa, T. 1990.
Cytolytic mechanism of activated macrophases. Tumor necrosis
factor and L-arginine-dependent mechanism acts as
synergistically as the mafor cytolytic mechanism of activated
macrophages. J Immunol 144, 1425-1431.
8. Tizard, I. R. and Schubot, R. M. 2004. Veterinary immunology
: An introduction. W. B. Saunders Company. U.S.
9. Hwang, J. S., Lee, J., Kim, Y. J., Bang, H. S., Yun, E. Y.,
Kim, S. R., Suh, H. J., Kang, B. R., Nam, S. H., Jeon, J. P.,
Kim, I. and Lee, D. G. 2009. Isolation and characterization
of a defensing-like peptide (Coprisin) from the dung beetle,
Copris tripartitus. Int J Pept 136.
10. Kang, B. R., Kim, H., Nam, S. H., Yun, E. Y., Kim, S. R.,
Ahn, M. Y., Chang, J. S., and Hwang, J. S. 2012. CopA3
peptide from Copris tripartitus induces apoptosis in human
leukemia cells via a caspase-independent pathway. BMB
Reports 45, 85-90.
11. Kang, J. K., Hwang, J. S., Nam, H. J., Ahn, K., Seok, J. H.,
and Kim, S. K. 2011. The insect peptide Coprisin prevents
Clostridium difficile-mediated acute inflammation and mucosal
damage through selective antimicrobial activity.
Antimicrob Agents Chemother 55, 4850-4857.
12. Kim, R. G., Shin, K. M., Chun, S. K., Ji, S. Y., Seo, S. H.,
Park, H. J., Choi, J. W. and Lee, K. T. 2002. In vitro anti-inflammatory
activity of the essential oil from ligularia fischeri
var. spiciformis in murine macrophage Raw 264.7 cells.
Yakhak Hoeji 46, 343-347.
13. Lee, Y. S., Kim, H. S., Kim, S. K. and Kim, S. D. 2000. IL-6
mRNA expression in mouse peritoneal macrophages and
NIH3T3 fibroblasts in response to Candida albicans. J
Microbiol Biotech 10, 9-15.
14. Lee, A. K., Sung, S. H., Kim, Y. C. and Kim, S. G. 2003.
Inhibition of lipopolysaccharide inducible nitric oxide synthase,
TNF-α and COX-2 expression by sauchinone effects
on I-κBα phosphorylation, C/EBP and AP-1 activation.
British J Pharmacol 139, 11-20.
15. Mori, M. 2007. Regulation of nitric oxide synthesis and apoptosis
by arginase and arginine recycling. J Nutr 137,
1616-1620.
16. Palmer, R. M., Ashton, D. S. and Moncada, S. 1988. Vascular
endothelial cells synthesize nitric oxide from L-arginine.
Nature 333, 664-666.
17. Park, D. S., Yoo, M. A., Xu, M. Z., Yu, H. N., Kim, J. R.,
Jeong, T. S. and Park, H. Y. 2004. Original articles : Screening
of anti-atherogenic substances from insect resources. Korean
J Pharmacogn 35, 233-238.
18. Park, K. T. and Lee, J. S. 1998. Review on insect resources
for medical use in kangwon Province. Korean J Apiculture
13, 79-92.
19. Suh, Y. J. 2002. Anti-tumor promoting potential of selected
spice ingredients with antioxidative and anti-inflammatory
activities.: A shor review. Food Chem Toxicol 40, 1091-1097.
20. Tezuka, Y., Irikawa, S., Kaneko, T., Banskota, A. H.,
Nagaoka, T., Xiong, Q., Hase, K. and Kadota, S. 2001.
Screening of chinese herbal drug extracts for inhibitory activity
on nitric oxide production and identification of an active
compound of zanthoxylum bungeanum. J Ethnopharmacol
77, 209-217.
21. Weisz, A., Cicatiello, L. and Esumi, H. 1996. Regulation of
the mouse inducible-type nitric oxide synthase gene promoter
by interferon-γ, bacterial lipopolysaccharide and
NG-monomethyl-L-arginine. Biochem J 316, 209-215.
22. Willoughby, D. A. 1975. Human arthritis applied to animal
models. Towards a beter therapy. Annals of the rheumatic
disease. Ann Rheum Dis 34, 471-478.
23. Won, S. J., Park, H. J. and Lee, K. T. 2008. Inhibition of
LPS induced iNOS, COX-2 and cytokines expression by slidroside
through the NF-κB inactivation in RAW 264.7 cells
Korean J Pharmacogn 39, 110-117.
24. Yun, H. J., Heo, S. K., Lee, Y. T., Park, W. H. and Park,
S. D. 2008. Anti-inflammatory effect of Evodia Officinalis
DODE in mouse macrophage and human vascular endotherial
cells. Korean J Herbology 23, 29-38.
|